Novel broad-spectrum treatments against tissue destructing cobra toxins
I have always seen myself as a natural born biologist with a great fascination for living creatures. Still, it wasn't until my PhD work that I got involved in the study of venomous animals. I began with scorpion research, but very soon, snakes won the contest and became the most remarkable venomous animals in my eyes. Snake venoms are highly complex and possess many fascinating bioactivities that may sometimes be used for beneficial purposes, although all too often snake venoms constitute a serious danger to human health. To me, it was shocking to find out how many people, especially those living in the rural parts of the world, are affected by these fantastic creatures' bites.
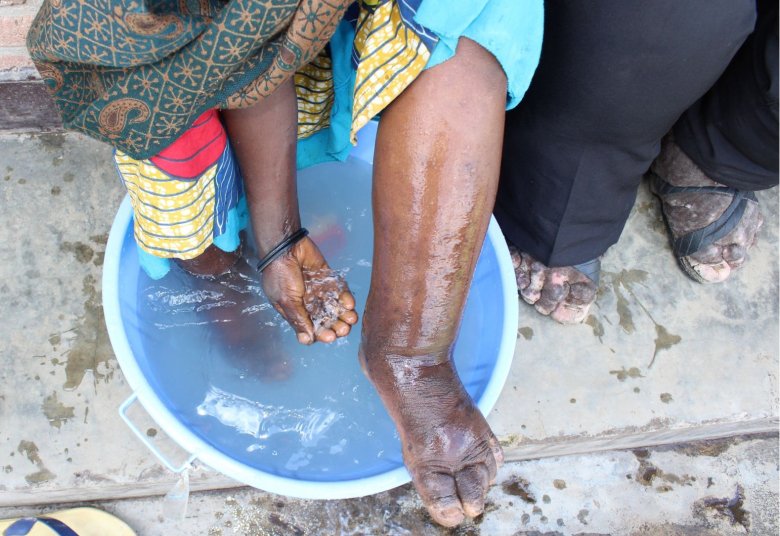
As a category A Neglected Tropical Disease, snakebite envenoming (SBE) annually claims tens of thousands of lives and leaves hundreds of thousands of permanently disabled people behind. Currently, SBE victims are treated with polyclonal antibodies derived from the plasma of immunized mammals, such as horses. Generally, these antivenoms are effective in saving lives, especially when they are administered shortly after the envenoming incidence. However, conventional antivenoms have some drawbacks including, but not limited to, the propensity of inducing adverse immune responses in victims due to their heterologous nature, as well as low efficacy in neutralizing locally acting toxins, such as cytotoxins (CTxs). As a consequence of the latter, many of the SBE victims who survive the venom's lethal effects still suffer from permanent disabilities, such as disfigurements or amputated limbs.


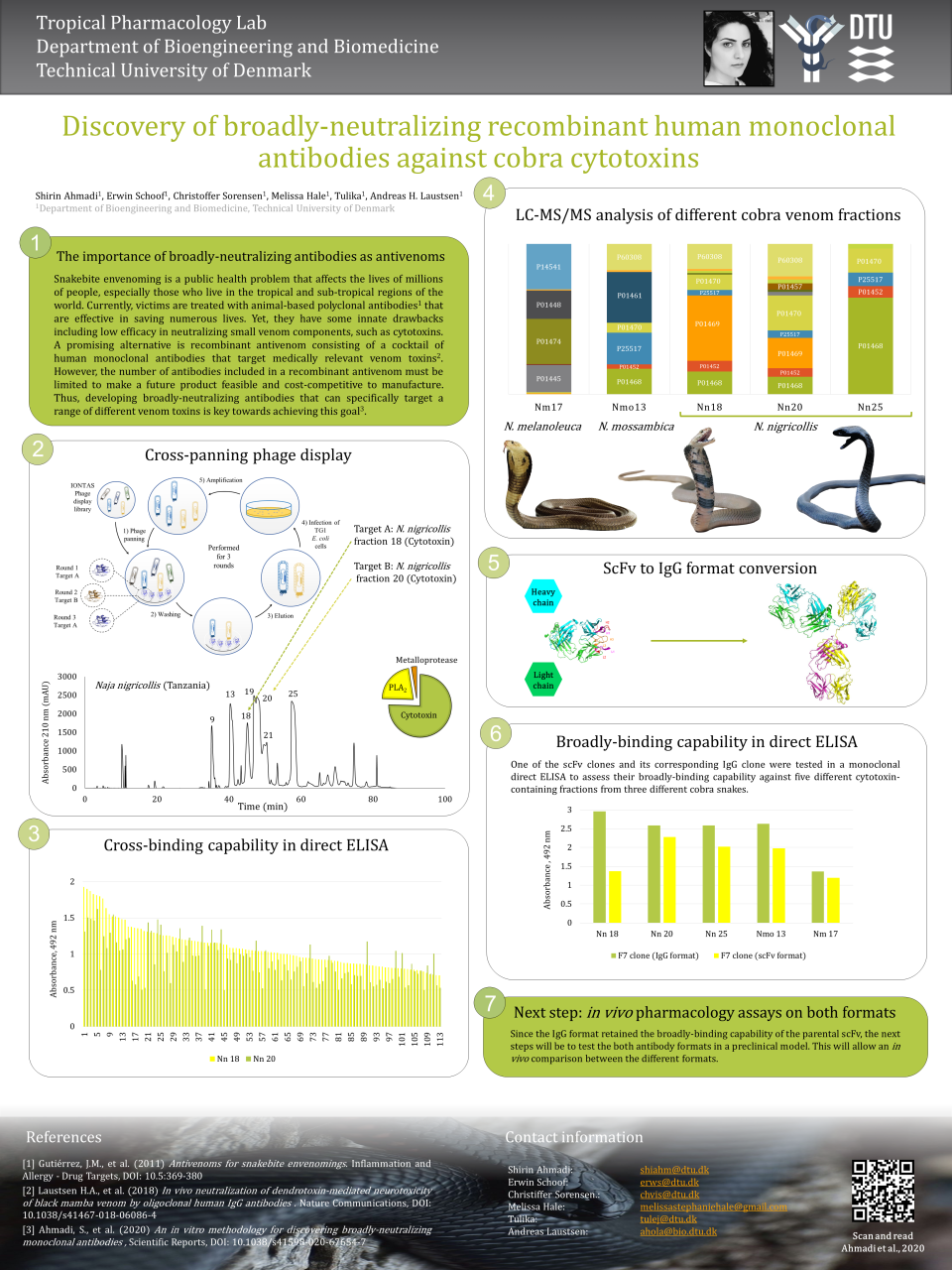
As a PhD student, I dedicated my research to find a solution to this problem. One of the promising solutions was the development of recombinant antivenoms containing cocktails of recombinant human monoclonal antibodies, where each monoclonal antibody is specifically selected to neutralize one or more medically relevant venom component (e.g., CTxs). However, the number of monoclonal antibodies included in the formulation of a recombinant antivenom must be limited to make them feasible to develop and cost-competitive to manufacture. Therefore, I developed an in vitro methodology for discovering monoclonal antibodies that target a broad spectrum of CTxs. Owing to this feature, higher numbers of different CTxs can be neutralized with fewer different antibodies.

In the poster that I virtually presented at the Venoms and Toxins 2020 conference, I demonstrated a schematic overview of the cross-panning phage display strategy that I implemented for discovering broadly-binding monoclonal antibodies. Using this strategy, I discovered over a hundred different antibodies in the single-chain variable fragment (scFv) format that could cross-bind to different CTx-containing venom fractions from the black-necked spitting cobra. Some of these scFvs also showed a capability to bind other CTx-containing venom fractions from other cobra snakes, such as the Mozambique cobra and forest cobra Thereafter, I converted one of the most promising broadly-binding scFvs to the whole immunoglobulin G (IgG) format and showed that the IgG retained the broadly-binding capability of the parental scFv clone. Therefore, my next step will be to test this antibody, in both the scFv and IgG formats, in an in vivo assay to see whether broad-binding capacity results in broad neutralization of different cobra CTxs.
I am positive that broadly-neutralizing, CTx-specific monoclonal antibodies can overcome the above-mentioned drawbacks of the conventional antivenoms. I hope that this study will help us get a step closer to developing an efficient, affordable treatment for the local effects caused by CTxs in cobra snakebites. In the future, this may help lower the number of SBE victims with life-long disabilities.