Wormy people: why some people are wormier than others
Ascariasis
Parasites are really interesting creatures, ranging from the deadly such as malaria, to the bizarre such as the Lancet liver fluke (which forces ants to climb blades of grass in order to be eaten by cows or sheep). Although describing symptoms of intestinal helminths isn’t always a great topic for a dinner party (depends on the party), I’m absolutely fascinated by them and am very fortunate to be spending four years studying one of the most common human parasites.
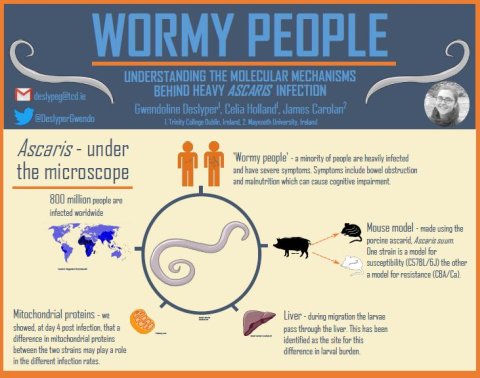
Ascariasis is a neglected tropical disease caused by the helminth Ascaris lumbricoides. Worldwide, 1 billion people are infected with this parasite with most cases occurring in Asia, Africa and South America.
Children between 5 and 15 often carry the heaviest worm burdens, which leads to chronic symptoms such as malnutrition and stunting. Severe acute symptoms, such as bowel obstruction and Loëffler syndrome, an allergic response to the migration of the larvae through the lungs, are quite rare.
Infection occurs through ingestion of the helminth egg, which contains infective larvae. As they reach the gut, the larvae hatch and migrate to the liver. From there they migrate to the lungs, get coughed up and are swallowed back in. The larvae will then mature into adult worms in the gut.
Different people, different worm burdens
Our lab in Trinity College Dublin, is particularly interested in a phenomenon known as aggregation, where some people become heavily infected and therefore get a heavy worm burden and others, are only lightly infected.
This remains true even after several rounds of treatment, where the same people keep getting the same heavy worm burden.
This recurrence of similar worm burden is called predisposition. It is often described as the 20/80 rule, where 20% of the population has 80% of the worm burden. Heavy worm burden is associated with more severe symptoms, making aggregation an important phenomenon to study.
We are currently investigating this difference in infection rate using different mouse strains, i.e. of the same genetic background.
In this model one strain, C57BL/6J, is susceptible to Ascaris infection and therefore a model for heavy infection. The other strain, CBA/Ca, is more resistant to Ascaris and is a model for light infection. The mice are infected with Ascaris suum, a porcine species of Ascaris.
Living it up in the liver
Our lab group has previously established that the difference in the level of infection occurs at the liver stage during the migration of the parasite.
The liver is a special organ when it comes to the immune system. It is immune tolerant, a feature first discovered during liver transplants. During these transplants it became clear that donor and receiver don’t have to be a perfect match.
This special immune status is a necessary feature for this organ, as during digestion food antigens reach the liver. If it wasn’t for this tolerance, an immune response would be activated with every meal.
This, however, has turned into a weak spot that can be exploited by parasites. Malaria, for example, reaches the liver soon after initial infection. This might be a strategy to play hide and seek from the host’s immune system. A similar mechanism could occur during Ascaris infection.
Current research
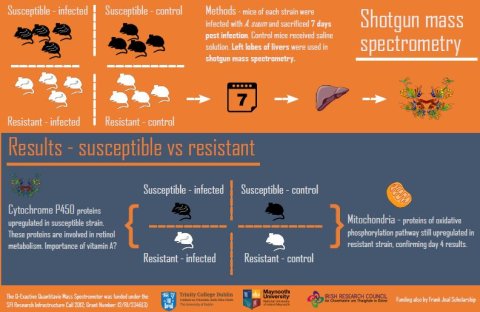
Using mass spectrometry, we have identified a number of proteins that are differentially expressed in the livers of the two mice strains during invasion of A. suum four days post-infection.
The results of that study were published in PLOS Neglected Tropical Diseases (Deslyper et al., 2016) and were pretty exciting. What struck us immediately was that there weren’t many proteins involved in the immune system present in our data, which was quite surprising.
This could be due to two reasons. Firstly, as discussed earlier, the liver is an immune tolerant organ and might not raise an immune response against the parasite. Ascaris is also known to excrete and secrete numerous proteins itself, which could also contribute to an immune modulation. Secondly, the time point of four days post infection could be too early to see an immune response.
We also found more mitochondrial proteins in the resistant strain than the susceptible strain both with and without infection. Mitochondria are the powerhouses of the cell and while they are great at producing energy, they also produce a side product called reactive oxygen species or ROS.
ROS often gets a bad reputation as being ‘toxic’, but in the case of infections, ROS can be beneficial and help fight off pathogens. This finding could, therefore, indicate that the resistant strain has more ROS and potentially a head start in contending with the parasite.
Additionally, we found a high abundance of S100a8 and S100a9 proteins under infection for both strains. These proteins form a heterodimer called calprotectin, which has been shown to play an important role in the defence against other parasites such as Leishmania, where this protein coats the parasite marking it for destruction by macrophages.
Therefore, the presence of these proteins could indicate that initiation of the immune system.
Another day, another insight
In a subsequent study we investigated the situation in the liver seven days post infection, again by using shotgun mass spectrometry. As with the earlier time point, we found that mitochondrial proteins were more abundant in the resistant strain than in the susceptible strain, both intrinsically and under infection.
We also found that proteins involved in retinol metabolism are more abundant in the susceptible strain compared to the resistant strain under infection. The role of retinol in Ascaris infection in humans has been studied numerous times, with conflicting results.
Some studies indicate that vitamin A supplementation to anthelmintic treatment decreases the reinfection rate compared to anthelmintic treatment alone.
However, similar studies found no statistical significance. Even though there is no conclusive evidence of the role of vitamin A, it is nevertheless interesting to find these proteins in our study.
In short, our research was able to identify important differences between the two mouse strains in mitochondrial and ribosomal proteins in addition to the retinol metabolism. These differences point us towards the solution of why some individuals get heavily infected and some don’t.
Baby steps first, giant leaps later
Our next steps in this project are to investigate how A. lumbricoides, the human ascarid, migrates through the body of our resistant and susceptible mice.
Subsequently, we will perform flow cytometry on the liver, in order to characterise the immune response in the liver.
This will increase our understanding of the observed difference in worm burden and will eventually aid the discovery of novel therapies.
Acknowledgments
This work is a collaboration between the parasitology lab in the Zoology department at Trinity College Dublin, Ireland, and the Applied Proteomics lab at Maynooth University, Ireland. Funding for this project comes from the Irish Research Council and the Frank Jeal Scholarship.